List of Publications
70. "Regio- and Chemoselective Palladium-Catalyzed Additive-Free Direct C─H Functionalization of Heterocycles with Chloroaryl Triflates Using Pyrazole-Alkyl Phosphine Ligands" Gu, C.; So, C. M.* Adv. Sci. 2024, DOI: 10.1002/advs.202309192.
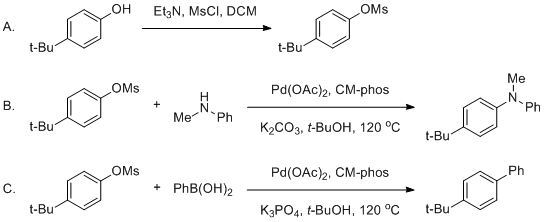
69. "Palladium-Catalyzed Chemoselective Amination of Chloro(hetero)aryl Triflates Enabled by Alkyl-Pyrazole-Based Phosphine Ligands" Gu, C.; Yuen, O. Y.; Ng, S. S.; So, C. M.* Adv. Synth. Catal. 2024, DOI: 10.1002/adsc.202301255. (Chosen as Very Important Publication and Front Cover)

68. "Palladium-Catalyzed Chemoselective Phosphorylation of Poly(pseudo)halides: A Route for Organophosphorus Synthesis" Chen, Z.‡; Pang, W. H.‡; Yuen, O. Y.; Ng, S. S.; So, C. M.* J. Org. Chem. 2024, DOI: 10.1021/acs.joc.3c02345. (Invited paper)
67. "Palladium-catalyzed chemoselective Suzuki–Miyaura cross-coupling reaction of poly(pseudo)halogenated arenes" Yuen, O. Y.‡; Ng, S. S.‡; Pang, W. H.; So, C. M.* J. Organomet. Chem. 2023, DOI: 10.1016/j.jorganchem.2023.122983. (Invited by Prof. Wai-Yeung Wong, Editor of the Journal of Organometallic Chemistry, this work is collected in the special issue titled "Organometallic Chemistry of Metal Clusters and Nanoparticles". This special issue dedicated to Prof. Wing-tak Wong on the occasion of his 60th birthday)
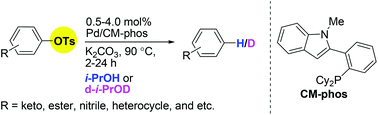
66. "Palladium-Catalyzed Deuterodehalogenation of Halogenated Aryl Triflates Using Isopropanol-d8 as the Deuterium Source" Miao, W.‡; Pang, W. H.‡; Yuen O. Y.; Ng, S. S.; So, C. M.* Org. Lett. 2023, 25, 8429-8433.
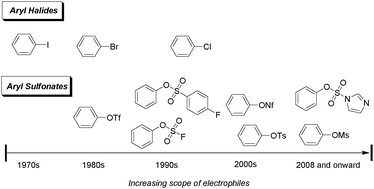
65. "Recent advances in the application of ligands in palladium-catalyzed chemoselective coupling reactions at C–Br, C–OTf, and C–Cl sites" Ng, S. S.‡; Pang, W. H.‡; Yuen, O. Y.; So, C. M.* Org. Chem. Front. 2023, 10, 4408-4436. (Invited by Deputy Editor of Organic Chemistry Frontiers, Prof. Kailin Deng , Chosen as Front Cover)
64. "Palladium-Catalyzed Desulfinative Cross-Coupling of Polyhalogenated Aryl Triflates with Aryl Sulfinate Salts: Inversion of Traditional Chemoselectivity" Wang, M.‡; Yuen, O. Y.‡; So, C. M.* Chin. J. Chem. 2023, 41, 909-914. (Invited by Guest Editor of Chinese Journal of Chemistry, Prof. Jun Wang (Joelle), Chosen as Front cover and highlighted in 中国化学Chin J Chem-"link" )
63. "Palladium-catalyzed desymmetric hydrophosphination of 3,3-disubstituted cyclopropenes" Yuen, O. Y.; So, C. M.* Chem Catal. 2022, 2, 2805-2807. (Invited by Scientific Editor of Chem Catalysis, Prof. Xiaoxiao Qiao)[link]
62. "Palladium-catalyzed chemoselective direct α-arylation of carbonyl compounds with chloroaryl triflates at the C–Cl site" Chen, Z.; Gu, C.; Yuen, O. Y.; So, C. M.* Chem. Sci. 2022, 13, 4658-4658. (Chosen as Inside front cover, and highlighted in RSC英国皇家化学会 - 钯催化羰基化合物与氯芳基三氟甲磺酸酯的化学选择性直接 α-芳基化反应 - "link")
61. "Palladium-Catalyzed Chemoselective Borylation of (Poly)halogenated Aryl Triflates and Their Application in Consecutive Reactions" Ng, S. S.; Chen, Z.; Yuen, O. Y.; So, C. M.* Adv. Synth. Catal. 2022, 364, 1596-1601. (Chosen as Very Important Publication and Front cover, and highlighted in 今日论文 - (多)卤代芳基三氟甲磺酸酯的钯催化化学选择性硼化反应及其在连续反应中的应用 - "link" )

60. "Palladium-Catalyzed Site-Selective Arylation of α,β-Unsaturated Carbonyl Compounds through a Ligand-Controlled Strategy" Yuen, O. Y.; So, C. M.* Synlett. 2022, DOI: 10.1055/s-0040-1719877. (Invited by Editor of Synlett, Prof. K. Peter C. Vollhardt.)  59. "An indole-amide-based phosphine ligand enabling a general palladium-catalyzed sterically hindered Suzuki–Miyaura cross-coupling reaction" Ng, S. S.; Chen, Z.; Yuen, O. Y.; So, C. M.* Org. Biomol. Chem. 2022, 20, 1373-1378.
59. "An indole-amide-based phosphine ligand enabling a general palladium-catalyzed sterically hindered Suzuki–Miyaura cross-coupling reaction" Ng, S. S.; Chen, Z.; Yuen, O. Y.; So, C. M.* Org. Biomol. Chem. 2022, 20, 1373-1378. 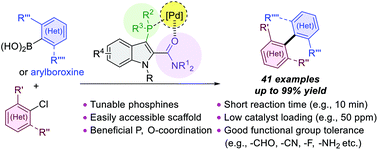

57. "General Chemoselective Suzuki–Miyaura Coupling of Polyhalogenated Aryl Triflates Enabled by an Alkyl-Heteroaryl-Based Phosphine Ligand" So, C. M.*; Yuen, O. Y.; Ng, S. S.; Chen, Z. ACS Catal. 2021, 11, 7820-7827.
56. "Palladium-Phenylpyrazolylphosphine-Catalyzed Cross-Coupling of Alkenyl Pivalates" Chen, Z.; So, C. M.* Asian J. Org. Chem. 2021, 10, 776-779. (Invited by Deputy Editor of Asian Journal of Organic Chemistry, Dr. Andrei Dragan & a special collection dedicated to early career researchers.)
55. "Design of Benzimidazolyl Phosphines Bearing Alterable P,O or P,N-Coordination: Synthesis, Characterization, and Insights into Their Reactivity" Wong, S. M.; Choy, P. Y.; Zhao, Q.; Yuen, O. Y.; Yeung, C. C.; So, C. M.; Kwong, F. Y. * Organometallics 2021, 40, 2265-2271.
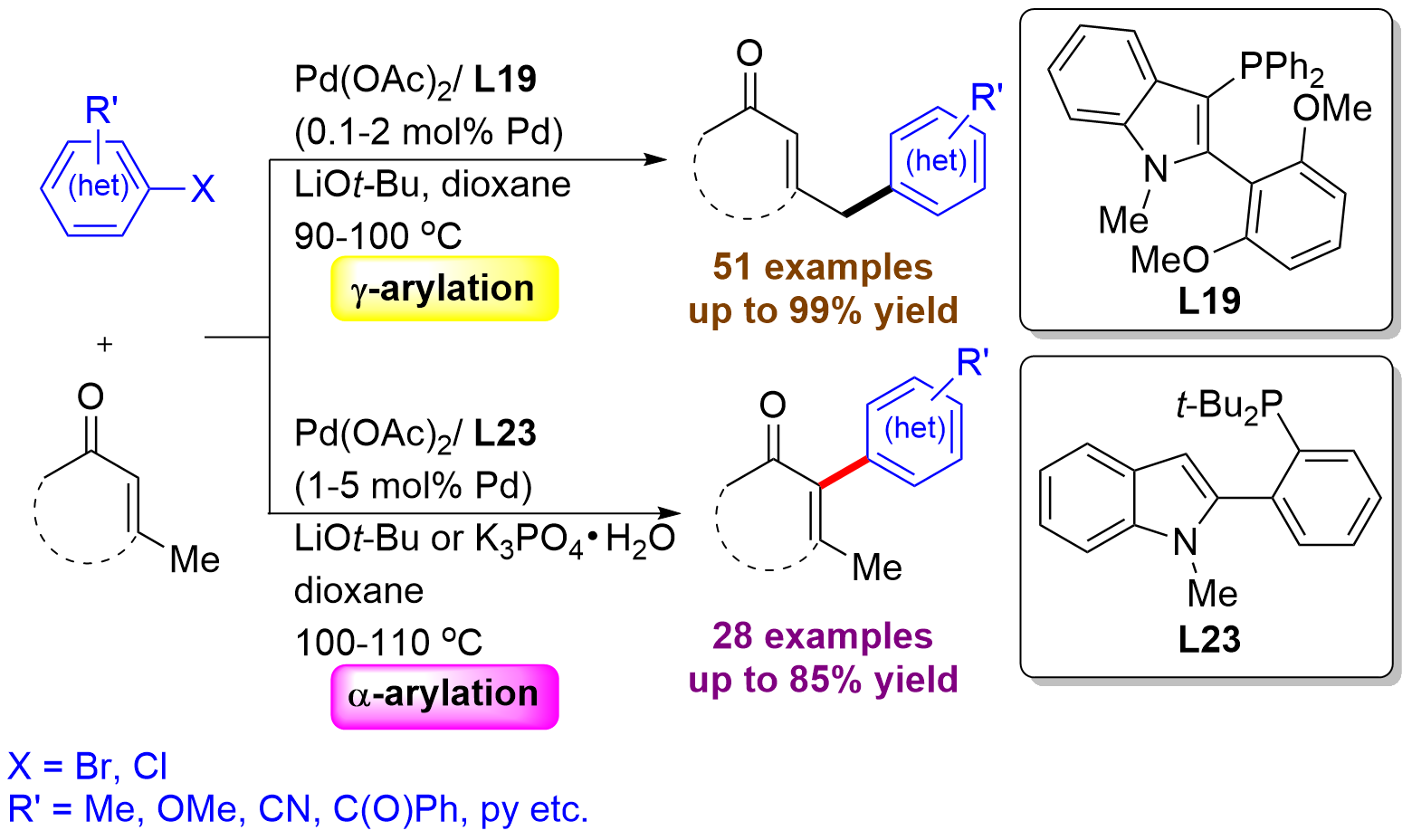
54. "A Ligand Control of Palladium‐Catalyzed Site‐Selective α‐ and γ‐Arylation of α,β‐Unsaturated Ketones with (Hetero)aryl Halides" Yuen, O. Y.; So, C. M.* Angew. Chem. Int. Ed. 2020, 59, 23438-23444. (Chosen as HOT Paper & Frontispiece & Highlighted in WileyChem - 配体控制的鈀催化位点选择性α,β-不饱和酮的芳基化反应 - "link")
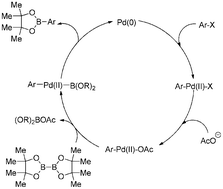
53. "Application of CM-Phos Ligand in Palladium-catalyzed Cross-coupling Reactions" So, C. M.*; Yuen, O. Y.; Kwong, F. Y.; Chen, C.; Pai, C; Sun, R. W. Y. Chemical Journal of Chinese Universities 2020, 41, 2185-2198. (Dedicated to Prof. Albert Sun-Chi Chan on the occasion of his 70th birthday) [link]
52. "Facile One-Pot Assembly of New 5-Substituted P,O-Type Indolylphosphine Ligands for Palladium-Catalyzed Suzuki-Miyaura Cross-Coupling of Aryl Chlorides" Leung, M. P.; Yeung, C C.; Choy, P. Y.; So, C. M.; Kwong, F. Y. . Chinese Journal of Organic Chemistry 2020, 40, 3338-3346. (Dedicated to Prof. Henry Nai Ching Wong on the occasion of his 70th birthday) [link]
51. "Pd-Catalyzed Cross-Coupling of Highly Sterically Congested Enol Carbamates with Grignard Reagents via C–O Bond Activation" Chen, Z.; So, C. M.* Org. Lett. 2020, 22, 3879-3883.
50. "Palladium-catalyzed cross-coupling of (hetero)aryl or alkenyl sulfonates with aryl titanium as the multi-functional reagent" Lee, H. W.‡; So, C. M.*‡; Yuen, O. Y.‡; Wong, W. T.*; Kwong, F. Y.* Org. Chem. Front., 2020,7, 926-932.
49. "Palladium‐Catalyzed Direct α‐Arylation of Arylacetonitriles with Aryl Tosylates and Mesylates" Yuen, O. Y.; Chen, X.; Wu, J.; So, C. M.* Eur. J. Org. Chem. 2020, 1912-1916. 
48. “Palladium-Catalyzed C(sp2)−N Bond Cross-Coupling with Triaryl Phosphates” Chen, Z.; Chen, X.; So, C. M.* J. Org. Chem. 2019, 84, 6366-6376.
47. “Exploration of Aryl Phosphates in Palladium-Catalyzed Mono-α-arylation of Aryl and Heteroaryl Ketones” Chen, X.; Chen, Z.; So, C. M.* J. Org. Chem. 2019, 84, 6337-6346.
46. “Synthesis of Flavone Derivatives through Versatile Palladium-Catalyzed Cross-Coupling Reactions of Tosyloxy- and Mesyloxyflavones” Yuen, O. Y.; Pang, W. H.; Chen, X.; Chen, Z.; Kwong, F. Y.; So, C. M.* Synlett 2019, 30, 731-737.
45. “Palladium-Catalyzed N-Arylation of Sulfoximines with Aryl Sulfonates” Yang, Q.; Choy, P. Y.; Zhao, Q.; Leung, M. P.; Chan, H. S.; So, C. M.; Wong, W. –T.; Kwong, F. Y. J. Org. Chem. 2018, 83, 11369-11376.
44. “A General Palladium–Phosphine Complex To Explore Aryl Tosylates in the N‐Arylation of Amines: Scope and Limitations” Choy, P. Y.; Chung, K. H.; Yang, Q.; So, C. M.*; Sun, R. W. –Y.; Kwong, F. Y. Chem. Asian. J. 2018, 13, 2465-2474.
43. “Palladium-Catalyzed Direct Arylation of Polyfluoroarenes for Accessing Tetra-ortho-Substituted Biaryls: Buchwald-type Ligand Having Complementary −PPh2 Moiety Exhibits Better Efficiency” Yuen, O. Y.; Leung, M. P.; So, C. M.*; Sun, R. W. –Y.; Kwong, F. Y. J. Org. Chem. 2018, 83, 9008-9017.
42. “Bulky Phosphane Ligand for Monoselective Ruthenium-Catalyzed, Directed o-C–H Arylation with Challenging Aryl Chlorides” Lu, Y. G.; Wang, Z. Y.; Zou, Y. L.; So, C. M.; Kwong, F. Y.*; Qin, H. L.; Kantchev, E. A.* Synlett 2017, 28, 499-503. (chosen as Cover)
41. “Catalytic Direct C2-Alkenylation of Oxazoles at Parts per Million Levels of Palladium/PhMezole-Phos Complex” Fu, W. C.; Wu, Y.; So, C. M.; Wong, S. M.; Lei, A.; Kwong, F. Y. Org. Lett. 2016, 18, 5300-5303.
40. “Exploiting Aryl Mesylates and Tosylates in Catalytic Mono-α-Arylation of Aryl- and Heteroarylketones” Fu, W. C.; So, C. M.; Yuen, O. Y.; Lee, I.; Kwong, F. Y. Org. Lett. 2016, 18, 1872-1875.
39. “A General Palladium-Catalyzed Hiyama Cross-Coupling Reaction of Aryl and Heteroaryl Chlorides” Yuen, O. Y.; So, C. M.*; Man, H. W.; Kwong, F. Y. Chem. Eur. J. 2016, 22, 6471-6476. (Chosen as HOT Paper & Inside Back Cover & Highlighted in the Chemistry Views)
38. “Open-Air Oxidative Mizoroki-Heck Reaction of Arylsulfonyl Hydrazides with Alkenes” Yuen, O. Y.; So, C. M.*, Kwong, F. Y. RSC Adv. 2016, 6, 27584-27589.
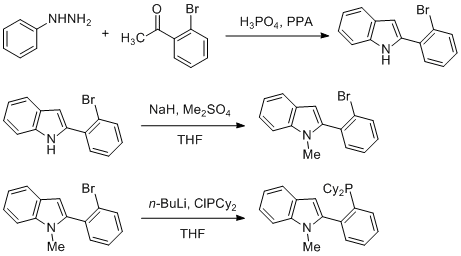
37. “Preparation of 2-(2-(dicyclohexylphosphino)phenyl)-1-methyl-1H-indole (CM-phos)” Wong, S. M.; Yuen, O. Y.; Choy, P. Y.; So, C. M.*; Kwong, F. Y. Org. Synth. 2016, 93, 14-28.
36. “Palladium-catalyzed Buchwald-Hartwig Amination and Suzuki-Miyaura Cross-coupling Reaction of Aryl Mesylates” Wong, S. M.; Choy, P. Y.; Yuen, O. Y.; So, C. M.*; Kwong, F. Y. Organic Syntheses 2015, 92, 195-212.
35. “Palladium-Catalyzed Phosphorylation of Aryl Mesylates and Tosylates” Fu, W. C.; So, C. M.; Kwong, F. Y. Org. Lett. 2015, 17, 5906-5909.

34. “Design of an Indolylphosphine Ligand for Reductive Elimination-Demanding Monoarylation of Acetone Using Aryl Chlorides” Fu, W. C.; So, C. M.*; Chow, W. K.; Yuen, O. Y.; Kwong, F. Y. Org. Lett. 2015, 17, 4612-4615.
33. “A General Direct Arylation of Polyfluoroarenes with Heteroaryl and Aryl Chlorides Catalyzed by Palladium Indolylphosphine Complexes” Yuen, O. Y.; Charoensak, M.; So, C. M.; Kuhakarn, C.; Kwong, F. Y. Chem. Asian. J. 2015, 10, 857-861.
32. “Rhodium/Chiral Diene-Catalyzed Asymmetric 1,4-Addition of Arylboronic Acids to Chromones: A Highly Enantioselective Pathway for Accessing Chiral Flavanones” He, Q.; So, C. M.; Bian, Z.; Hayashi, T.; Wang, J.* Chem. Asian. J. 2015, 10, 540-543.
31. “A Chiral Bicyclo[2.2.2]octa-2,5-diene Ligand Substituted with the Ferrocenyl Group and Its Use for Rhodium-Catalyzed Asymmetric 1,4-Addition Reactions” Zhou, B.; So, C. M.; Lu, Y.*; Hayashi, T.* Org. Chem. Front. 2015, 2, 127-132.
30. “Regioselective Direct C-3 Arylation of Imidazo[1,2‑a]pyridines with Aryl Tosylates and Mesylates Promoted by Palladium−Phosphine Complexes” Choy, P. Y.; Luk, K. C.; Wu, Y.; So, C. M.; Wang, L.*; Kwong, F. Y.* J. Org. Chem. 2015, 80, 1457-1463.
29. “A General Suzuki-Miyaura Coupling of Aryl Chlorides with Potassium Aryltrifluoroborates in Water Catalyzed by an Efficient CPCY Phendole-phos-Palladium Complex” Yuen, O. Y.; Wong, S. M.; Chan, K. F.; So, C. M.*; Kwong, F. Y. Synthesis 2014, 46, 2826-2832.
28. “Palladium-Catalyzed Reductive Cleavage of Tosylated Arenes Using Isopropanol as the Mild Reducing Agent” Chow, W. K.; So, C. M.; Lau, C. P.; Kwong, F. Y. Org. Chem. Front. 2014, 1, 464-467.
27. “Rhodium-Catalyzed Asymmetric Hydroarylation of 3-Pyrrolines Giving 3-Arylpyrrolidines: Protonation as a Key Step” So, C. M.; Satoshi, K.; Hayashi, T.* J. Am. Chem. Soc. 2013, 135, 10990-10993.
26. “A Decade Advancement of Transition Metal-Catalyzed Borylation of Aryl Halides and Sulfonates” Chow, W. K.; Yuen, O. Y.; Choy, P. Y.; So, C. M.; Lau, C. P.; Wong, W. T.; Kwong, F. Y. RSC Adv. 2013, 3, 12518-12539.
25. “Direct Oxidative C-H Arylation of Benzoxazoles with Arylsulfonyl Hydrazides Promoted by Palladium Complexes” Yuen, O. Y.; So, C. M.; Wong, W. T.; Kwong, F. Y. Synlett 2012, 23, 2714-2718.
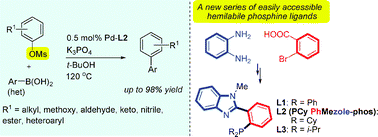
24. “An Efficient Class of P,N-Type "PhMezole-phos" Ligands: Applications in Palladium-Catalyzed Suzuki Coupling of Aryl Chlorides” Wong, S. M.; So, C. M.*; Chung, K. H.; Lau, C. P.; Kwong, F. Y. Eur. J. Org. Chem. 2012, 22, 4172-4177.
23. “P,N-Type Benzimidazolyl Phosphine Ligands for the Palladium-Catalyzed Suzuki Coupling of Potassium Aryltrifluoroborates and Aryl Chlorides” Wong, S. M.; So, C. M.*; Chung, K. Ho; L., C. H.; Lau, C. P.; Kwong, F. Y. T. Lett. 2012, 53, 3754-3757.
22. “Buchwald-Hartwig Amination of Aryl Chlorides Catalyzed by Easily Accessible Benzimidazolyl Phosphine-Pd Complexes” Chung, K. H.; So, C. M.*; Wong, S. M.; Luk, C. H.; Zhou, Z.; Lau, C. P.; Kwong, F. Y. Synlett 2012, 23, 1181-1186.
21. “The Recent Development of Phosphine Ligands Derived from 2-Phosphino-Substituted Heterocycles and Their Applications in Palladium-Catalyzed Cross-Coupling Reactions” Wong, S. M.; So, C. M.; Kwong, F. Y. Synlett 2012, 23, 1132-1153.
20. “Carbon-Boron Bond Cross-Coupling Reaction Catalyzed by –PPh2 Containing Palladium-Indolylphosphine Complexes” Chow, W. K.; Yuen, O. Y.; So, C. M.; Wong, W. T.; Kwong, F. Y. J. Org. Chem. 2012, 77, 3543-3548.
19. “Palladium-Catalyzed Direct Arylation of Polyfluoroarenes with Aryl Tosylates and Mesylates” Lee, D. S.; Choy, P. Y.; So, C. M.; Wang, J.; Lau, C. P.; Kwong, F. Y. RSC Adv. 2012, 2, 9179-9182.
18. “An Efficient Palladium–Benzimidazolyl Phosphine Complex for the Suzuki–Miyaura Coupling of Aryl Mesylates: Facile Ligand Synthesis and Metal Complex Characterization” Chung, K. H.; So, C. M.*; Wong, S. M.; Luk, C. H.; Zhou, Z.; Lau, C. P.; Kwong F. Y. Chem. Commun. 2012, 48, 1967-1969.
17. “Palladium-Catalyzed Borylation of Aryl Mesylates and Tosylates and Their Applications in One-Pot Sequential Suzuki-Miyaura Biaryl Synthesis” Chow, W. K.; So, C. M.; Lau, C. P.; Kwong, F. Y. Chem. Eur. J. 2011, 17, 6913-6917.
16. “A Mild and Efficient Palladium-Catalyzed Cyanation of Aryl Chlorides with K4[Fe(CN)6]” Yeung, P. Y.; So, C. M.; Lau, C. P.; Kwong, F. Y. Org. Lett. 2011, 13, 648-651.
15. “Hydro(trispyrazolyl)borato-Ruthenium(II) Diphosphinoamino Complex- Catalyzed Addition of β-Diketones to 1-Alkynes and Anti-Markovnikov Addition of Secondary Amines to Aromatic 1-Alkynes” Cheung, H. W.; So, C. M.; Pun, K. H.; Zhou, Z.; Lau, C. P. Adv. Synth. Catal. 2011, 353, 411-425.
14. “Palladium-Catalyzed Direct Arylations of Heteroarenes with Aryl Mesylates” So, C. M.; Lau, C. P.; Kwong, F. Y. Chem. Eur. J. 2011, 17, 761-765.
13. “Palladium-Catalyzed Cross-Coupling Reactions of Aryl Mesylates” So, C. M.; Kwong, F. Y. Chem. Soc. Rev. 2011, 40, 4963-4972.
12. “A Versatile Palladium Catalyst System for Suzuki–Miyaura Coupling of Alkenyl Tosylates and Mesylates” Wong, P. Y.; Chow, W. K.; Chung, K. H.; So, C. M.; Lau. C. P.; Kwong, F. Y. Chem. Commun. 2011, 47, 8328–8330.
11. “Palladium-Catalyzed Sonogashira Coupling of Aryl Mesylates and Tosylates” Choy, P. Y.; Chow, W. K.; So, C. M.; Lau, C. P.; Kwong, F. Y. Chem. Eur. J. 2010, 16, 9982-9985.
10. “A General Palladium Catalyst System for Suzuki-Miyaura Coupling of Potassium Aryltrifluoroborates and Aryl Mesylates” Chow, W. K.; So, C. M.; Lau, C. P.; Kwong, F. Y. J. Org. Chem. 2010, 75, 5109-5112.
9. “Remarkably Effective Phosphanes Simply with a PPh2 Moiety: Application to Pd-Catalysed Cross-Coupling Reactions for Tetra-ortho-substituted biaryl Syntheses” So, C. M.; Chow, W. K.; Choy, P. Y.; Lau, C. P.; Kwong, F. Y. Chem. Eur. J. 2010, 16, 7996-8001.
8. “A Mild and Efficient Palladium-Catalyzed Cyanation of Aryl Mesylates in Water or tBuOH/Water” Yeung, P. Y.; So, C. M.; Lau, C. P.; Kwong, F. Y. Angew. Chem. Int. Ed. 2010, 122, 9102-9106. (chosen as Hot Paper)
7. “Palladium-Indolylphosphine-Catalyzed Hiyama Cross-Coupling of Aryl Mesylates” So, C. M.; Lee, H.W.; Lau, C. P.; Kwong, F. Y. Org. Lett. 2009, 11, 317-320.
6. “Palladium-Catalyzed Cross-Coupling of Aryl Halides Using Organotitanium Nucleophiles” Lee, H. W.; Lam, F. L.; So, C. M.; Lau, C. P.; Chan, A. S. C.; Kwong, F. Y. Angew. Chem. Int. Ed. 2009, 121, 7572-7575.
5. “A New Family of Tunable Indolylphosphine Ligands by One-Pot Assembly and Their Applications in Suzuki-Miyaura Coupling of Aryl Chlorides” So, C. M.; Yeung, C. C.; Lau, C. P.; Kwong, F. Y. J. Org. Chem. 2008, 73, 7803-7806.
4. “Suzuki-Miyaura Coupling of Aryl Tosylates Catalyzed by an Array of Indolyl Phosphine-Palladium Catalysts” So, C. M.; Lau, C. P.; Chan, A. S. C.; Kwong, F. Y. J. Org. Chem. 2008, 73, 7731-7734.
3. “A General Palladium-Catalyzed Suzuki–Miyaura Coupling of Aryl Mesylates” So, C. M.; Lau, C. P.; Kwong, F. Y. Angew. Chem. Int. Ed. 2008, 47, 8059-8063.
2. “Palladium-Catalyzed Amination of Aryl Mesylates” So, C. M.; Zhou, Z.; Lau, C. P.; Kwong, F. Y. Angew. Chem. Int. Ed. 2008, 47, 6402-6406.
1.“Easily Accessible and Highly Tunable Indolyl Phosphine Ligands for Suzuki-Miyaura Coupling of Aryl Chlorides” So, C. M.; Lau, C. P.; Kwong, F. Y. Org. Lett. 2007, 9, 2795-2798.
Wong, S. M.; So, C. M.*; Kwong, F. Y. Ligand-Enabled Palladium-Catalyzed C-N and C-O Bond Formations from Aryl Halides, Tosylates and Mesylates. In Applied Homogeneous Catalysis with Organometallic Compounds; Cornils, B., Herrmann, W. A., Beller, M., Paciello, R., Eds.; Wiley-VCH: Weinheim, 2017; pp 435-464.
 Patents:
Patents:
1. Kwong, F. Y.; So, C. M. Ligands for Transition-Metals and Methods of Use. US Patent 8212056-B2.
2. 邝福儿, 周永健, 苏秋铭, 蔡珮盈 "高位阻芳基硼酸酯类化合物的制备方法" CN104327106B.
3. 邝福儿, 蔡珮盈, 苏秋铭, 原安莹 "(2﹣二取代膦苯基)-1-烷基-吲哚膦配体及其合成方法和应用" CN104945434B.
4. 邝福儿, 苏秋铭, 周永健, 原安莹 "一种吲哚骨架的膦配体及其制备方法和应用" CN107445989B.
5. 邝福儿, 傅伟聪, 杜尚俊, 苏秋铭, 蔡珮盈 “咔唑基磷配体、及其制备方法和应用” CN106588983B.
6. 苏秋铭; 原安莹; 吴珊珊; 陈梓聪 "一种2-烷基-吲哚骨架的膦配体及其制备方法和应用" CN113402553B.
7. 苏秋铭; 陈梓聪 "5-(2-(二取代膦基)苯基)-1-烷基-1H-吡唑膦配体及其制备方法和应用" CN114907404B.
8. 苏秋铭; 原安莹 "一种吡唑-酰胺骨架的膦配体及其制备方法与应用" CN115703806B.



























![Graphical abstract: A chiral bicyclo[2.2.2]octa-2,5-diene ligand substituted with the ferrocenyl group and its use for rhodium-catalyzed asymmetric 1,4-addition reactions](https://pubs.rsc.org/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C4QO00292J&imageInfo.ImageIdentifier.Year=2015)